Inarigivir soproxil
CAS No. 942123-43-5
Inarigivir soproxil( SB-9200 | SB9200 | Inarigivir | ORI-9020 )
Catalog No. M16738 CAS No. 942123-43-5
Inarigivir soproxil (SB-9200, SB9200, Inarigivir, ORI-9020) is an orally bioavailable dinucleotide that activates RIG-I and NOD2, induces interferon mediated antiviral immune responses.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 356 | Get Quote |


|
| 10MG | 530 | Get Quote |


|
| 25MG | 851 | Get Quote |


|
| 50MG | 1152 | Get Quote |


|
| 100MG | 1557 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameInarigivir soproxil
-
NoteResearch use only, not for human use.
-
Brief DescriptionInarigivir soproxil (SB-9200, SB9200, Inarigivir, ORI-9020) is an orally bioavailable dinucleotide that activates RIG-I and NOD2, induces interferon mediated antiviral immune responses.
-
DescriptionInarigivir soproxil (SB-9200, SB9200, Inarigivir, ORI-9020) is an orally bioavailable dinucleotide that activates RIG-I and NOD2, induces interferon mediated antiviral immune responses; shows broad-spectrum antiviral activity against RNA viruses including HCV, norovirus, RSV and influenza, and also demonstrates activity HBV in vitro and in vivo; a novel agonist of innate immunity, and prodrug of Inarigivir (ORI-9020;SB-9000).HBV Infection Phase 2 Clinical(In Vitro):Inarigivir soproxil (SB 9200) is a first-in-class oral modulator of innate immunity that acts via the activation of the RIG-I and NOD2 pathways.Inarigivir soproxil is an effective inhibitor of HCV replication in cell culture. The antiviral activity of Inarigivir soproxil against HCV was assessed using genotype 1 HCV replicon systems in duplicate experiments using 4 drug concentrations. Inarigivir soproxil inhibits HCV replication with EC50s of 2.2 and 1.0 μM, and EC90s of 8.0 and 6.0 μM for genotype 1A and 1B, respectively.Inarigivir soproxil (SB 9200), an orally bioavailable dinucleotide, activates the viral sensor proteins, retinoic acid-inducible gene 1 (RIG-I) and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) causing the induction of the interferon (IFN) signaling cascade for antiviral defense.(In Vivo):The induction of host immune responses by pretreatment with Inarigivir soproxil (SB 9200) followed by Entecavir (ETV) resulted in antiviral efficacy that was superior to that obtained using the strategy of viral reduction with ETV followed by immunomodulation.Sequential treatment of chronic WHV carrier woodchucks with Inarigivir soproxil (30 mg/kg)followed by ETV induced marked suppression of serum viremia and antigenemia and delayed recrudescence of viral replication compared to sequential treatment with ETV followed by Inarigivir soproxil.
-
In VitroInarigivir soproxil (SB 9200) is a first-in-class oral modulator of innate immunity that acts via the activation of the RIG-I and NOD2 pathways. Inarigivir soproxil is an effective inhibitor of HCV replication in cell culture. The antiviral activity of Inarigivir soproxil against HCV was assessed using genotype 1 HCV replicon systems in duplicate experiments using 4 drug concentrations. Inarigivir soproxil inhibits HCV replication with EC50s of 2.2 and 1.0 μM, and EC90s of 8.0 and 6.0 μM for genotype 1A and 1B, respectively.Inarigivir soproxil (SB 9200), an orally bioavailable dinucleotide, activates the viral sensor proteins, retinoic acid-inducible gene 1 (RIG-I) and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) causing the induction of the interferon (IFN) signaling cascade for antiviral defense.
-
In VivoThe induction of host immune responses by pretreatment with Inarigivir soproxil (SB 9200) followed by Entecavir (ETV) resulted in antiviral efficacy that was superior to that obtained using the strategy of viral reduction with ETV followed by immunomodulation.Sequential treatment of chronic WHV carrier woodchucks with Inarigivir soproxil (30 mg/kg)followed by ETV induced marked suppression of serum viremia and antigenemia and delayed recrudescence of viral replication compared to sequential treatment with ETV followed by Inarigivir soproxil. Animal Model:Woodchucks chronically infected with woodchuck hepatitis virus (WHV)Dosage:30 mg/kg Administration: Orally treated once daily; group 1 received ETV for 4 weeks followed by Inarigivir soproxil for 12 weeks. Group 2 received Inarigivir soproxil for 12 weeks followed by ETV for 4 weeks Result:Both groups demonstrated marked reductions in hepatic WHV nucleic acid levels which were more pronounced in Group 2.
-
SynonymsSB-9200 | SB9200 | Inarigivir | ORI-9020
-
PathwayImmunology/Inflammation
-
TargetRLR
-
RecptorRLR
-
Research AreaInfection
-
Indication——
Chemical Information
-
CAS Number942123-43-5
-
Formula Weight703.617
-
Molecular FormulaC25H34N7O13PS
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
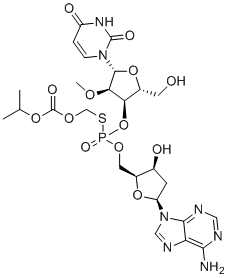
SMILESO[C@@H](C[C@@H](N1C2=C(C(N)=NC=N2)N=C1)O3)[C@@H]3COP(O[C@@H]4[C@@H](CC)O[C@@H](N5CCC(NC5=O)=O)[C@@H]4OC)(SCOC(OC(C)C)=O)=O
-
Chemical Name(((((2S,3S,5S)-5-(6-amino-9H-purin-9-yl)-3-hydroxytetrahydrofuran-2-yl)methoxy)(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-(hydroxymethyl)-4-methoxytetrahydrofuran-3-yl)oxy)phosphoryl)thio)methyl isopropyl carbonate
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Korolowicz KE, et al. PLoS One. 2016 Aug 23;11(8):e0161313.
2. Suresh M, et al. PLoS One. 2017 Jan 5;12(1):e0169631.
3. Jones M, et al. J Med Virol. 2017 Sep;89(9):1620-1628.
4. Iyer RP, et al. Antimicrob Agents Chemother. 2004 Jun;48(6):2318-20.
molnova catalog



related products
-
KIN-1408
A small-molecule MAVS-dependent IRF3 agonist that shows antiviral activity against Flaviviridae.
-
KIN-1400
A potent RIG-I-like receptor (RLR) agonist that triggers IRF3-dependent innate immune antiviral genes and IFN-β expression.
-
Inarigivir
Inarigivir (ORI-9020, SB-9000) is a dinucleotide that activates RIG-I and NOD2.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


